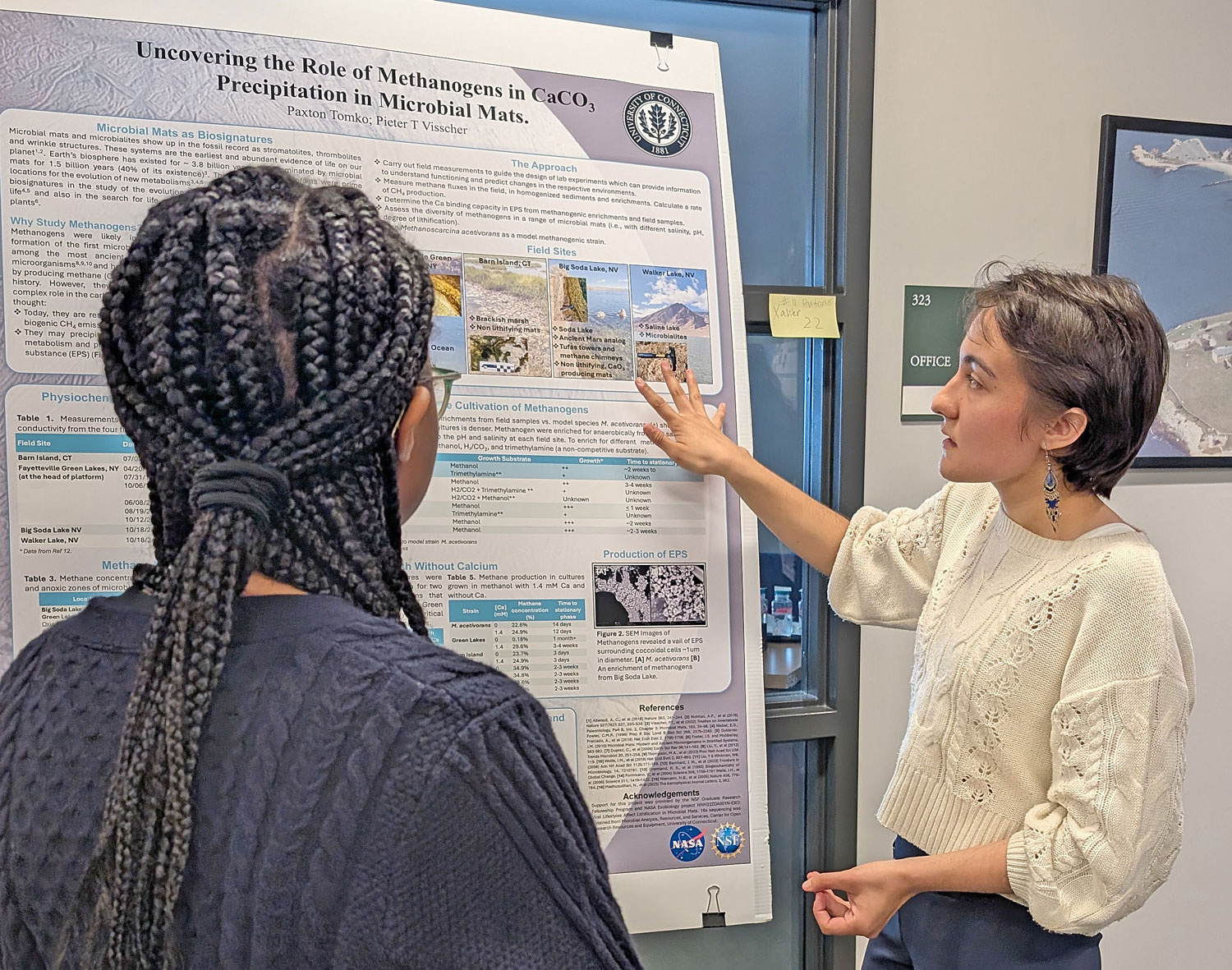
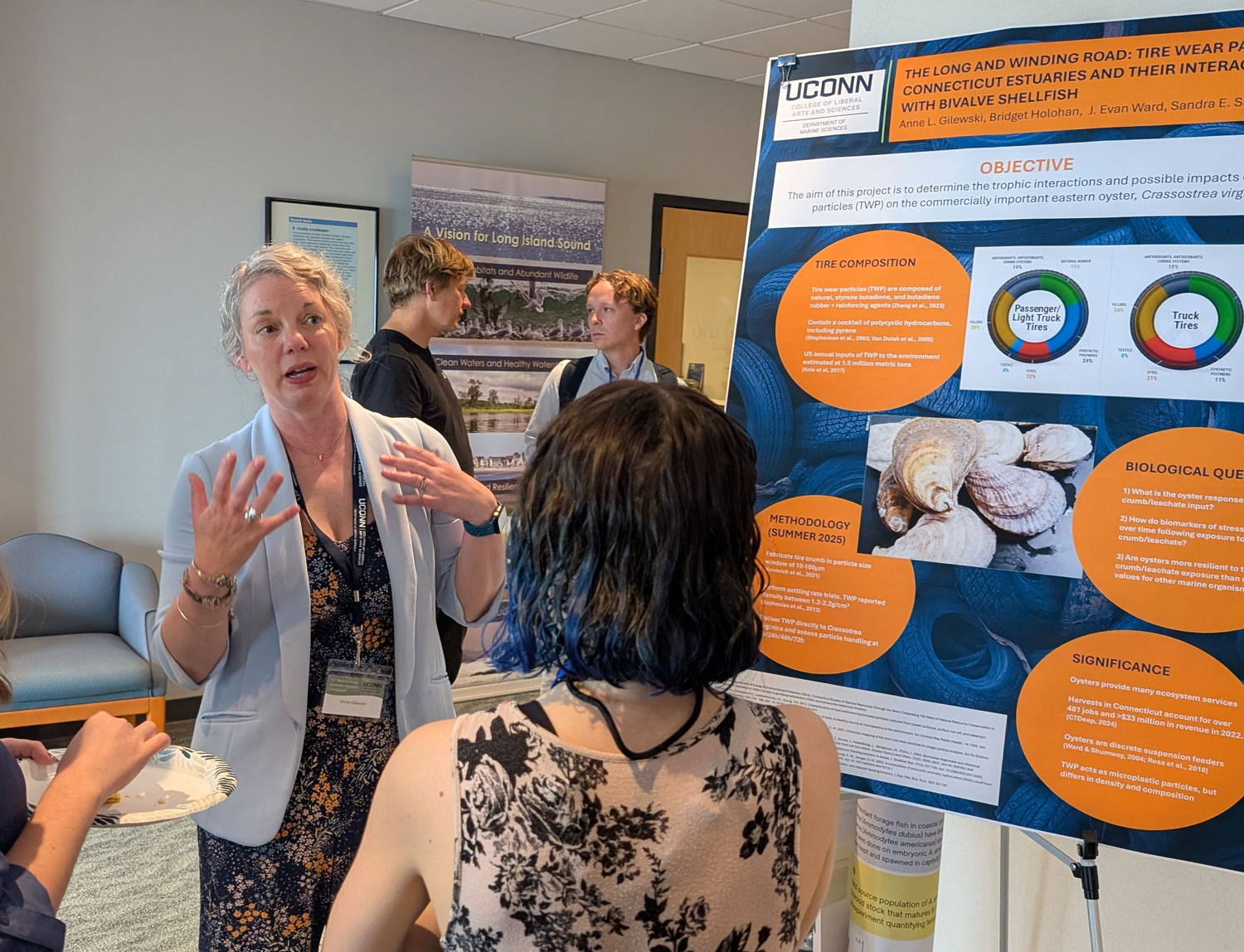
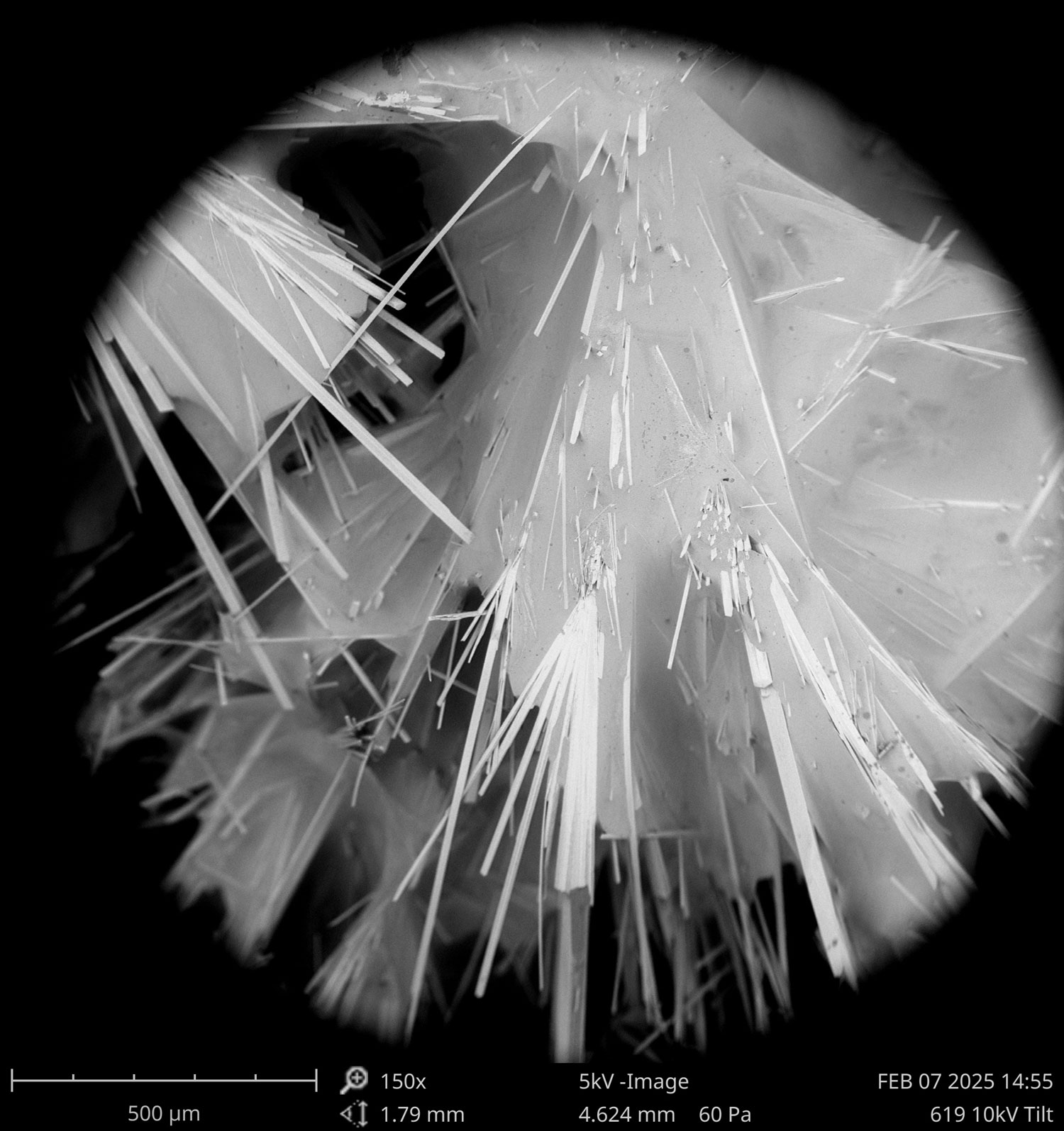
Please join us in congratulating Prof. Pieter Visscher for receiving the Geological Society of America’s 2025 Distinguished Career Award (sponsored by the Geobiology and Geomicrobiology Division).
Pieter is honored for his decade-long involvement in an NSF-sponsored project that has worked with undergraduate students studying microbial sediments in Puerto Rico. Between 2000 and 2011, Pieter brought 68 of these students to Avery Point for a two-week geomicrobiology short course. None of these first-generation college students had visited the continental US before. His continued encouragement inspired over 60 students in this program to pursue graduate degrees in the US. He taught this course twice in France, once in Argentina, and once in Chile.
Pieter is further commended for his pioneering Astrobiology work, pursuing the fundamental questions: Where are we coming from, where are we going, and what is our future? His tireless curiosity ultimately led to the establishment of NASA’s Astrobiology Institute (NAI) in 1998; the product of a four-decade-long collaboration with scholars, scientists, and engineers from around the country.
We asked Pieter to name a key paper from the many published in his career - he chose his 2020 study of arsenotrophic microbial mats that allow inferring our past:
Visscher P.T., K.L. Gallagher, A. Bouton, M.E. Farias, D. Kurth, B.P. Burns, M.R. Walter, M. Sancho-Tomas, P. Philippot, A. Somogyi, K. Medjoubi, E. Vennin, R. Bourillot, M. Contreras, C. Dupraz. 2020. Modern arsenotrophic microbial mats provide an analogue for life in the anoxic Archean. Nature Communications Earth & Environment 1:24
Citation
by Tracy Frank:
Pieter T. Visscher. Over a distinguished career spanning more than three decades, Dr. Visscher has led advances in our understanding of the complex interactions between microbial communities, biogeochemical cycles, and the formation of sedimentary structures, bridging microbiology and Earth system science. His pioneering studies of microbial mats and stromatolites across a range of settings have illuminated the role of microbes in shaping Earth’s surface environments through time, while his innovative approaches to microbial processes in modern and ancient settings have inspired new directions in research. His atmospheric biosignature studies were instrumental for NASA’s Astrobiology Institute, of which he was a co-founding member. A prolific scholar and respected mentor, Dr. Visscher has authored hundreds of influential publications and trained generations of graduate students who continue to advance the discipline worldwide. His research, collaborations, and leadership have had a transformative impact on geomicrobiology, leaving a legacy that will guide the science for decades to come.
Reply
Pieter: Thank you, Tracy, for your kind citation. I am honored to receive this recognition, which has been previously awarded to geochemists, paleontologists, sedimentologists, and geobiologists for whom I have great respect.
As many know, the term geobiology was coined by Lourens G.M. Baas Becking, but not in his monograph “Geobiologie”, published in 1934, but in his inaugural lecture at the University of Leiden on January 28, 1931, entitled “Gaia of leven en aarde”. In this lecture, Baas Becking observed a then recent change in natural and physical scientific research that for the first time were deployed jointly to understand our planet. Furthermore, he expanded his observation that chemistry, biology, and geology did not just apply to the understanding of our planet Earth but provided the foundation for understanding the universe, which he argued was fueled by cyclic events - notably chemical element cycles, but also (micro)biological metabolisms, and physical phenomena, the mutual impact of which was captured in geological time and space. In a way, this laid the groundwork for what, 65 years later, became a motivation for NASA’s Pale Blue Dot II meeting that, in turn, started Astrobiology. Baas Becking viewed geobiology as a Copernican-based science, in other words, perceived not from an anthropocentric, Earth-centric viewpoint, but from a geocentric one. What really matters is that geo(micro)biology is a transdisciplinary science, not just a multidisciplinary one. The answer to “big questions” in this discipline is best solved by first assessing which disciplinary tools are needed, not just by combining disciplinary views that address the question. Despite incredible advances in methodologies in the last four decades, each approach has its limitations. This is critical to remember.
My educational background is based on a combination of organic chemistry and the Delft School of Microbiology – that, in addition to Baas Backing, also included Beijerinck, Kluyver, and van Niel. In addition to having a great mentor, Hans van Gemerden, who combined detailed observations and measurements in the field with meaningful ecophysiological laboratory experiments, I have had the good fortune to meet many scientists who have shaped our discipline as we know it today. The list is quite exhaustive, but I would like to mention a handful of them here briefly, some unsung heroes in geomicrobiology, in chronological order.
Wolfgang Krumbein was instrumental during my PhD work on sulfur cycling at the University of Groningen. After a long 24 hours of fieldwork, being in a group that was the first to use microelectrodes in the field during diel cycles, a very tedious process at the time, he would sit me down in his cigar-smoke-filled office and question me about everything I had observed and was planning to do as follow-up. Early in my grad student years, he sent me a box with well over a hundred of his reprints. Using geology and microbiology, his strong belief that the laminae in stromatolites are the same as those in microbial mats may be wrong, but it nevertheless challenged our thinking and steered us in the right direction to better understand the role of microbes on early Earth and throughout geologic time. Spending three weeks with Dick Castenholz and Bev Pierson in Yellowstone was a life-changing event. The vast diversity of environments in a relatively small area was an eye-opener. Likewise, several long discussions, both in the field and during elegant home-cooked meals with Malcolm Walter, have contributed much to advancing my work. Lynn Margulis, who, by the way, together with James Lovelock reintroduced the term Gaia in 1974, 43 years after Baas Becking, Ed Leadbetter, Ron Oremland, Jack Farmer, and Dave DesMarais were all instrumental as post-doc and early career mentors. Generous scientists who gave selfless advice and listened. I learned a lot from them and expanded my scientific and interpersonal horizons. It takes a village….
I would like to thank the people who are keeping our field alive: the leaders of the professional societies, notably the Division of Geobiology and Geomicrobiology of Geological Society of America, the reviewers of our proposals and manuscripts, the program directors at funding agencies, the educators, who sparked the interest in students who then come as graduate assistants or post-docs to our labs, and all those students and colleagues with whom I had many fruitful/stimulating discussions and rewarding collaborations. I am excited about the direction in which our field is moving, and hope that we jointly continue to combine the many subdisciplines that make up geo(micro)biology in a meaningful way to unlock the secrets that our field holds in shaping our planet and the many worlds beyond.