A supply grant from UConn's Office of Undergraduate Research (OUR) will test whether cyanobacteria could assist with removing carbon dioxide
Evelyn Lewis glances at the well plates full of colorful slime in Prof. Visscher’s lab and smiles. The life thriving in there is invisible to the naked eye, but she knows how to keep the microscopic critters happy. For almost a year now, she has helped taking care of them, and this has helped others in the lab with their research projects.
But now, Evelyn is starting a project of her own. Her soft voice betrays the nascent excitement, as she examines a well plate full of what looks like crusty, white dust.
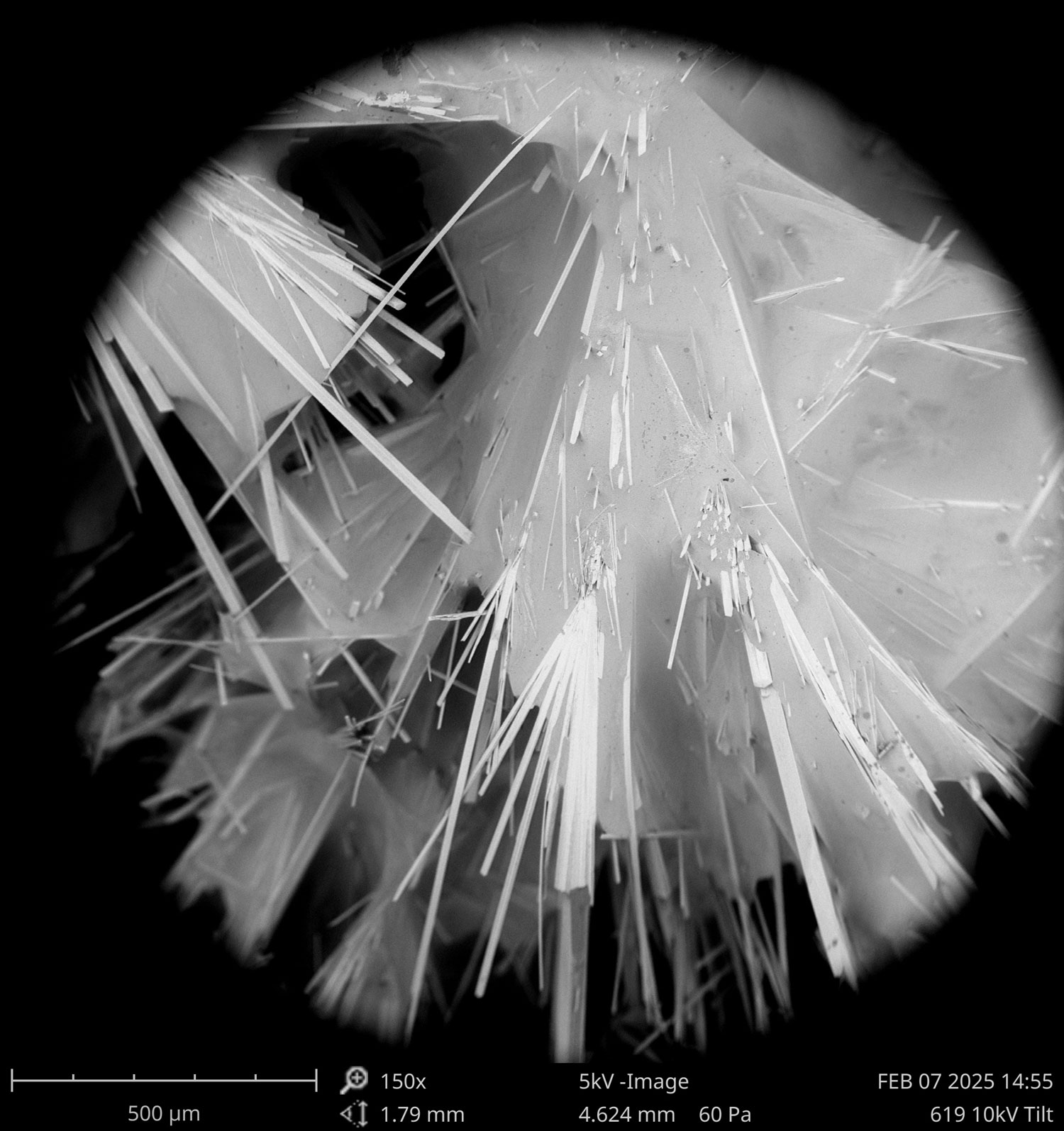
“These are calcium carbonate crystals, and they look so beautiful under the microscope”, she says.
Thanks to a new supply grant from UConn’s Office for Undergraduate research, she will now have the opportunity to look at many more of these crystals. Evelyn’s research will focus on some of the smallest photosynthetic organisms in world, cyanobacteria. When they bloom they often coat themselves in slime that they can chemically manipulate. The conditions in this extracellular slime might then become favorable to bind carbon dioxide (CO2) in form of calcium carbonate (CaCO3), ultimately removing it from the atmosphere. In other words, cyanobacteria may be tiny but mighty as a natural tool for combating the increase of heat-trapping CO2 in the atmosphere.
“These natural options of using microbial slime for CO2 removal remain surprisingly underexplored”, explains Visscher. “The slime binds calcium and when it sinks to the bottom, it supports CaCO3 formation in sediments for thousands of years. This recently discovered mechanism provides novel insights into the global carbon cycle.”
So over the course of the next months, Evelyn will culture cyanobacteria again – but this time for her project. In small well plates, she will measure their CaCO3 production for about two weeks in relation to differing amounts of calcium. Yet the arguably coolest part will come after that, when the collected crystals will be examined using scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS).
Ultimately, the gathered data will allow testing the overarching hypothesis that the presence of cyanobacteria increases CaCO3 precipitation.