It’s finally springtime in the Northeast USA, so gardeners are looking to fertilize their plants and flowers. Have you ever stopped to wonder what is actually in the big bags of soil you spread on your lawn?
In most cases, it is a mixture of nitrogen and phosphorus. Both are limiting nutrients for the base of the food chain, aka plants. Phosphorus makes its way into soil and the ocean through weathering of rocks. Nitrogen, on the other hand, is trickier, because although it makes up over 70% of the atmosphere, this gaseous form (N2) is unusable by plants and animals.
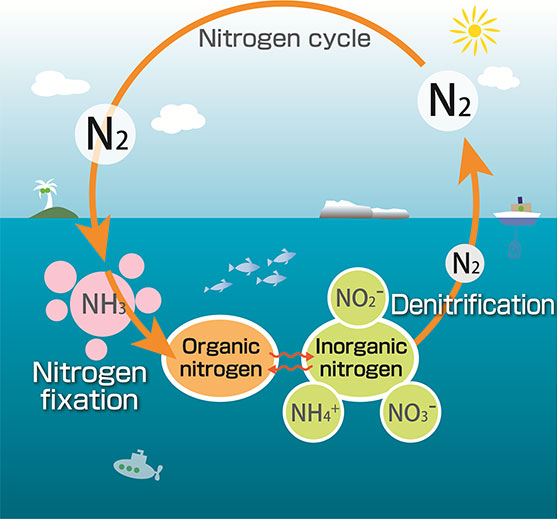
So how does nitrogen get from the atmosphere into a usable form? The process is called “nitrogen fixation.” On land, bacteria in soil do the heavy lifting by converting N2 into organic nutrients like ammonium (NH4+) and nitrate (NO3–) that are usable by plants. In the ocean, blue-green cyanobacteria are the most abundant type of bacteria to fix nitrogen. Collectively, these organisms are called diazotrophs, and account for close to 90% of natural nitrogen fixation.
In a recent collaborative publication, Associate Professor Julie Granger and Professor Craig Tobias contributed their expertise on the nitrogen cycle in the ocean. The study included scientists from various institutions and focused on standardizing procedures for measuring diazotrophic activity in field sample incubations.
Oceanographers are interested in understanding the magnitude and rate of nitrogen fixation by diazotrophs. These rates vary significantly depending on the location, whether coastal or open ocean, poles or equator, shallow or deep. Ultimately, scientists aim to evaluate what controls the reservoir of nitrogen in the ocean from measurements and its influence on ocean fertility. Unfortunately, researchers currently employ differing techniques, causing uncertainty in whether estimates of nitrogen fixation rates are inter-comparable among research groups and among ecosystems.
Granger, Tobias and colleagues focus on the 15N2 tracer method for this study. Therein, water samples from a particular depth and region of the ocean are incubated and supplemented with isotopically-labeled nitrogen gas (15N2 gas).
Isotopes refer to the different masses an element can have depending on its atomic structure. Nitrogen can have atomic mass of 14 or 15, which are referred to as 14N or 15N. Naturally occurring nitrogen is predominantly 14N, such that adding a dollop of 15N can facilitate the tracking of nitrogen transformations.
During incubation, living diazotrophs in the water samples convert nitrogen gas (including the labeled 15N-gas) into nitrogen nutrients. At the end of the incubation, the isotopic composition (whether 14N or 15N) of the newly “fixed” nitrogen is measured. This method allows scientists to track the amount and rate of atmospheric nitrogen gas that was converted into nitrogen nutrients at a particular ocean location.
Over the years, renditions of the 15N2 tracer method have been conceived, including some with questionable practices. As not all are created equal, Granger, Tobias and co-authors ultimately recommend the so-called “dissolution” and the “bubble release” methods over others.
Additionally, Granger and Tobias stress the importance of adhering to proven mass spectrometric procedures to quantify 15N, which is crucial for obtaining representative estimates.
The paper thus states, “While the research community may remain divided as to which variant of the method to follow, the standardization of some key practices will enable intercomparability among estimates, to better discern temporal and biogeographical trends, as well as environmental controls on ocean N2 fixation.”
While both Granger and Tobias are proud of the resulting document, they agree that they will never again engage in the Herculean effort that is achieving consensus: “Herding cats is harder than science.”

Citation:
White, A.E., Granger, J., Selden, C., Gradoville, M.R., Potts, L., Bourbonnais, A., Fulweiler, R.W., Knapp, A.N., Mohr, W., Moisander, P.H., Tobias, C.R., Caffin, M., Wilson, S.T., Benavides, M., Bonnet, S., Mulholland, M.R. and Chang, B.X. (2020), A critical review of the 15N2 tracer method to measure diazotrophic production in pelagic ecosystems. Limnol Oceanogr Methods. doi:10.1002/lom3.10353