
By Mengyang Zhou
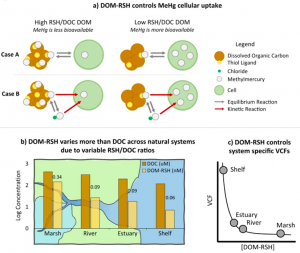
Connecticut and New England, like many locations around the world, houses many estuaries that suffer from mercury pollution that is largely inorganic, less toxic and not as bioaccumulative as methylmercury (MeHg). The mercury concentrations in these regions are much higher than in the open ocean despite only a small fraction of these concentrations being bioavailable. Eventually, inorganic Hg is converted to MeHg and enters the food web. Coastal regions, like estuaries, also support marine resource cultivation and supply to communities, and therefore are potentially a source of exposure for humans to elevated levels of MeHg. A key environmental control determining MeHg bioavailability is dissolved organic matter (DOM), and specifically the sulfur-containing thiol binding ligands within the DOM, as they strongly bind to MeHg. Understanding how dissolved organic matter (DOM) influences MeHg bioavailability is crucial for predicting exposure in high-trophic level biota, including humans. Environmental changes, such as eutrophication and altered runoff, impact DOM loading in aquatic ecosystems and therefore can affect MeHg bioavailability. Historically, using dissolved organic carbon (DOC) as a proxy for MeHg[RM1] -binding capacity has been a standard, but this work shows this assumption may lead to errors depending on the natural environment you are working in. The group hypothesized that the properties of DOM unrelated to total DOC may be impacting MeHg bioavailability, and may underpin the variability in MeHg uptake at low DOC concentrations commonly observed in the environment. Specifically, the group tested whether DOM binding capacity, defined as the concentration of thiol ligands per gram DOC, or the DOM binding strength to MeHg dictated overall MeHg bioavailability in distinct coastal regions.
To address this, the team measured DOM properties associated with MeHg bioavailability across four distinct regions of the nearshore terrestrial-marine aquatic continuum. They found that the in situ MeHg-binding capacity of DOM varied significantly and systematically across the terrestrial-marine aquatic continuum they explored, but the binding affinity varied but not significantly or systematically across the same system, and ligand exchange kinetics were fast for all types of DOM.
Next, they explored the DOM principles they highlight as driving MeHg uptake by testing it in a phytoplankton uptake experiment using a specific species of diatom. Their results supported that not only the total DOM concentration but also the concentration of specific DOM-associated sulfur compounds called thiol functional groups binding sites (DOM-RSH) were the primary factors controlling the MeHg bioavailability and accumulation within the phytoplankton.
The project was started by Emily Seelen PhD ’18 (Fig. 1), now a postdoctoral researcher at the University of Southern California, when she was a graduate researcher in Professor Robert Mason’s lab (Fig. 2). Pf. Mason’s funded projects supported the work and Seelen also received an NSF graduate fellowship that enabled a study abroad collaboration with co-authoring chemists Erik Björn, Ulf Skyllberg, and Van Liem-Nguyen from Umeå University in Sweden. Seelen was also advised by Associate Research Professor Zofia Baumann who instructed Seelen on how to perform the phytoplankton uptake experiments, drawing from her previous research tracing heavy metal contaminants through the environment.
Seelen said she was inspired to pursue this work in part as an opportunity to work abroad as a result of her research with Professor Mason. “With Rob I was able to learn a lot about broad coastal Hg cycling, but I knew I wanted to dive a bit deeper into what exactly controlled how much MeHg was able to enter into the food web. I put together a list of potential universities I could study at, and soon got to meet Erik Bjorn at the 2015 Hg conference (ICMGP) in Korea. I instantly knew this was the working group for me and we wrote the proposal soon after!” She then proceeded to apply for the NSF graduate fellowship to pursue this idea and was successful in acquiring one of these highly competitive fellowships.
The study emphasizes the crucial role of dissolved organic matter (DOM) in MeHg bioaccumulation in aquatic ecosystems. It reveals that MeHg uptake by phytoplankton is directly linked to the DOM-RSH rather than the more traditional binding calculation for MeHg -dissolved organic carbon (DOC) interaction. Figure 3 showcases how decreasing trends in DOM-RSH concentrations across the terrestrial to marine aquatic continuum leads to higher MeHg availability and planktonic uptake in systems dominated by marine DOM relative to those impacted by more humic, terrestrial DOM. The research suggests that measuring DOM-RSH concentrations is essential for accurate models in understanding MeHg incorporation in aquatic food webs across different environments. The research highlights the need to consider specific DOM characteristics, specifically the ratio of thiol functional groups to DOC (DOM-RSH/DOC), for more accurate predictions under various environmental scenarios.
https://www.nature.com/articles/s41467-023-42463-4
Link to UConn Today article