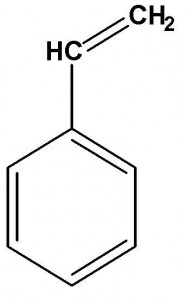
 The image to the left shows the tankship IEVOLI SUN sinking in English Channel in October 2000 carrying 4000 tons of styrene monomer. The image to the right shows the chemical formula of monomeric styrene.
The image to the left shows the tankship IEVOLI SUN sinking in English Channel in October 2000 carrying 4000 tons of styrene monomer. The image to the right shows the chemical formula of monomeric styrene.
In the past three decades, the environmental fates of many volatile organic compounds (VOCs) in marine waters have been thoroughly investigated. Aromatic hydrocarbons in particular have been examined in various laboratory microcosms and at on-site monitoring stations in order to model the transport of these compounds in seawater. Despite this wide-ranging research, however, one commonly used industrial chemical has received little attention in regards to its impact and eventual degradation when spilled into aquatic systems. Styrene monomer is an aromatic hydrocarbon extensively used for making plastic, fiberglass, and styrofoam products. The high demand for this clear, oily liquid has created a market where thousands of tons of styrene are produced and shipped over water around the world. Consequently, the risks of large aquatic spills of this chemical have steadily increased.
When exposed to certain chemical initiators, heat, or ultraviolet light, styrene monomer will polymerize into a solid mass termed polystyrene. Under controlled conditions, this quality makes styrene very useful for making products ranging from packing material to compact disk cases. Although the solid polymer after polymerization is not considered hazardous, the liquid monomer is toxic to aquatic life and has been classified as a possible carcinogen. These and other issues have raised concerns about the chemical especially during large spill events such as a barge collision in Louisiana in 1992 and the recent sinking of the tankship IEVOLI SUN in the English Channel in 2000.
Although most styrene will evaporate in a large spill, several questions still exist as to how the remaining styrene will cycle through the aquatic environment. Our laboratory is exploring other spill fates of the chemical including sorption and biodegradation processes. In addition, we are reviewing possible spontaneous polymerization processes of styrene during seawater spills. This research will provide many useful answers for emergency responders and scientists in understanding and cleaning up future spills of this important industrial chemical. The research will also help to develop general environmental-fate models that may be applied to other chemicals.